Society has long fantasized about a day when science would provide technological cures for societal ills such as aggression, impulsive decision-making, and depression. In the popular science fiction TV Show, Star Trek, a medical tricorder was waved over the body, magically probing internal systems and recalibrating problems without any side effects. For some medical illnesses, such a device seems just around the corner (See Qualcomm’s $10 Million Tricorder XPrize; Scanadu Scout). However, for mental illnesses, which are particularly complex and poorly understood, such a solution remains elusive. Nevertheless, several prominent media outlets have drawn attention to the use of transcranial direct current stimulation (tDCS), a technique which delivers a low-intensity direct current to modulate the activity of neurons in the cerebral cortex, as an early example of such a fabled device.

Indeed, by utilizing tDCS, researchers at academic medical centers have made widespread reports of its therapeutic effects on a number of neuropsychiatric disorders ranging from major depressive disorder, pain disorders, musculoskeletal disorders, drug addiction, Parkinson’s disease and motor deficits after stroke. Moreover, outside a disease population, researchers have also found that a normal population is capable of benefitting from tDCS – showing increased performance across a variety of cognitive tasks such as attention, memory and decision-making.
Perhaps what is most striking about tDCS is its capability to achieve all this as a noninvasive procedure with limited side effects. As a result, tDCS holds the potential to be a safe and effective alternative to pharmacological intervention in patients with complicated medical histories whose drug regimen flags a contraindication for standard therapy. Additionally, the accepted safety of tDCS makes it appealing for treating certain populations, such as pregnant women suffering from a depressive disorder. And lastly, there is the low cost, simplicity and portability of the tDCS medical device itself. In many cases, the unit is just a 9-volt battery with a strip of electrodes that adhere to the patients’ scalp, costing well under $100 to produce.
So how does tDCS work? One theory of its mechanism of action is that tDCS affects critical processes that underpin the brain’s neuroplasticity. Specifically, researchers believe that tDCS influences the behavior of NMDA receptors, which play a crucial role in the molecular basis of learning and memory, through their role in coincidence detection and long term potentiation. In addition, tDCS is thought to affect the firing rates of neurons by increasing their excitability through an altered resting membrane potential.
Despite the recent clamor over tDCS, it is fundamental to recognize that electrically stimulating the brain has a long and rich history in medicine. Indeed, electrically stimulating the brain can be traced to the storied physicians of antiquity, like Galen, who once proposed that an electric current, generated by a fish, could relieve a headache. In the 18th century, the development of batteries enabled early researchers such as Giovanni Aldini and Luigi Galvani to elicit varying physiological effects by applying current to the brain, such as triggering muscle contractions in the deceased by stimulating the corpus callosum. While these early experiments underscored the brain as an electrically excitable organ, it was not until the turn of the 21st century that the application of current to stimulate the cerebral cortex would be investigated again to explore its psychiatric effects.
Still, scientific and clinical interest in modulating neuronal activity to cure disease continued unabated – with the 20th century heralding the development of several FDA-approved neurotherapeutic technologies still in use today. Electroconvulsive therapy (ECT), developed in the early 20th century, is now a well-tolerated treatment in the management of severe psychiatric illness, including schizophrenia and major depression. While the exact mechanism of ECT remains unknown, ECT therapy involves inducing a seizure by electrically stimulating one or both hemispheres of the patient’s brain with a strong electric current. One major drawback of ECT is its lack of specificity: a patient’s entire brain is stimulated. Moreover, ECT can cause cognitive side effects and memory loss, possibly as the result of this poor specificity.
Transcranial magnetic stimulation (TMS) has emerged over the past two decades as a safer ECT in a sense, stimulating a smaller area of the brain and with less propensity for causing cognitive side effects. Like ECT, TMS is a noninvasive way to manipulate brain circuits. However, TMS operates by the electromagnetic induction of an electric field in the brain, allowing for the modulation of neuronal activity. However, since the stimulation can be localized to a population of neurons 1.0-3.0 cm beneath the scalp, TMS does not carry the significant side effect profile of ECT. And while early phase version of this device suffered from controversies regarding its potential to induce seizures, TMS obtained FDA approval in 2008 for the treatment of unipolar depression resistant to pharmacological intervention and is now even being reimbursed for by insurance companies.
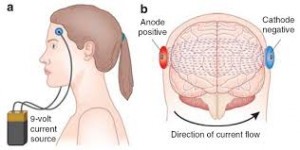
The DIY version of tDCS requires just a 9-volt battery and some wire.
Whereas TMS requires expensive equipment and a clinical setting, the affordability, portability and low cost of tDCS has already led to the early adoption by private individuals and for-profit companies, despite the lack of FDA approval. In fact, the attention tDCS has received in the media has generated so much interest that London-based manufacturer, Foc.us, sold out of its initial run of prefabricated tDCS units each priced at $250. And yet, directly marketing a medical technology in the early stages of development to consumers carries with it many concerns that should be addressed before the technology gains mainstream use. For instance, few end users may realize that what’s harmless in the hands of experts could nonetheless have risks when performed by consumers.
As tDCS continues to be developed and these safety concerns are addressed, as has been the case with ECT and TMS, tDCS may carry unique ethical and regulatory challenges. For instance, who should be licensed to use this technology? What degree of training should be required? Should the use of tDCS to enhance the cognitive function of healthy adults be permitted? If so, what could be the consequences of unequal access to this treatment in society?
If ongoing research continues to suggest the benefits of tDCS outweigh these long-term concerns, one can imagine the benefits this technology could bring to society: less depression, less aggression, workers who are more alert, more productive, and happier. The big question is whether as a society, the slippery slope of personal enhancement leads to a better society, or a dystopic future that changes what it means to be human.